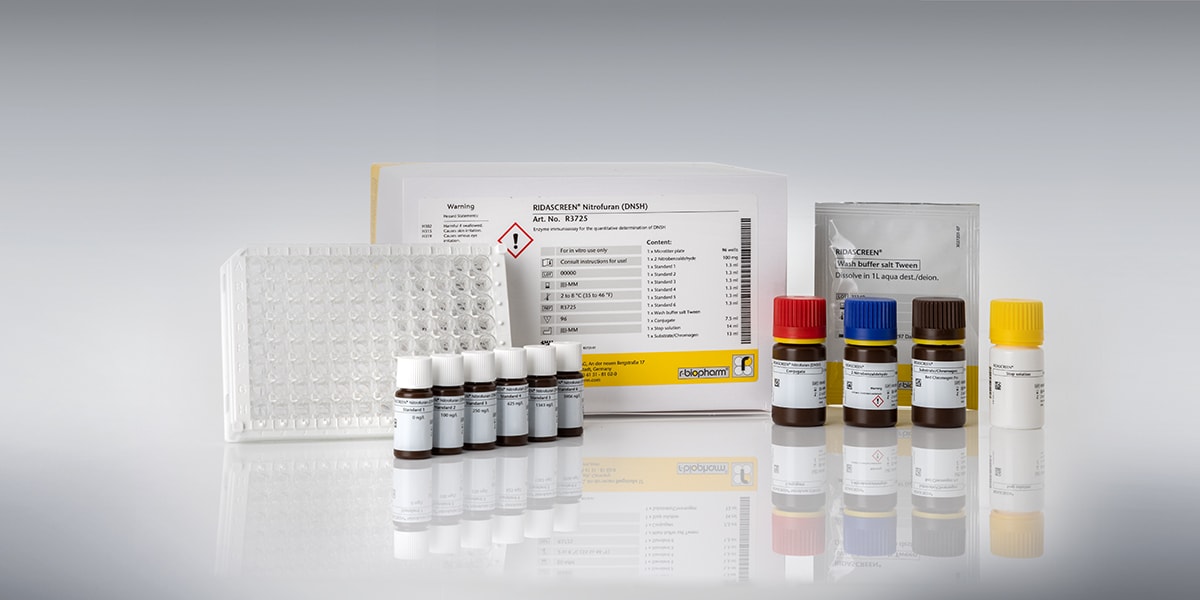
RIDASCREEN® Nitrofuran (DNSH)
Art. No. R3725
Product info about RIDASCREEN® Nitrofuran (DNSH)
Intended use
RIDASCREEN® Nitrofuran DNSH (Art. No. R3725) is a competitive enzyme immunoassay for the quantitative determination of the Nifursol metabolite 3,5-dinitrosalicylic acid hydrazide (DNSH) in shrimp.
General Information
Nifursol is a nitrofuran antibiotic that is banned as a feed additive in the European Union and other countries. Nifursol is metabolized in living organisms to 3,5-dinitrosalicylic acid hydrazide (DNSH). DNSH is a marker for detecting the illegal use of nifursol in livestock. Detection of DNSH by LC-MS/MS requires derivatization of this metabolite with 2-nitrobenzaldehyde to NP-DNSH, as with other nitrofuran metabolites such as SEM, AHD, AMOZ and AOZ. The antibody used in this assay detects DNSH without derivatization with 2-nitrobenzaldehyde. According to Commission Regulation (EU) 2019/1871, a reference point for action (RPA) of 0.5 µg/kg for DNSH and other nitrofuran metabolites will apply from November 28th, 2022.
Accessories
Dear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | R3725 |
| Test format | Microtiter plate with 96 wells (12 strips with 8 individual wells each) |
| Sample preparation | homogenization and extraction |
| Validated matrices | Shrimp |
| Detected analyte | 3,5-Dinitrosalicylic acid hydrazide (DNSH) in shrimp |
| Evaluation | Microtiter plate photometer (450 nm) |