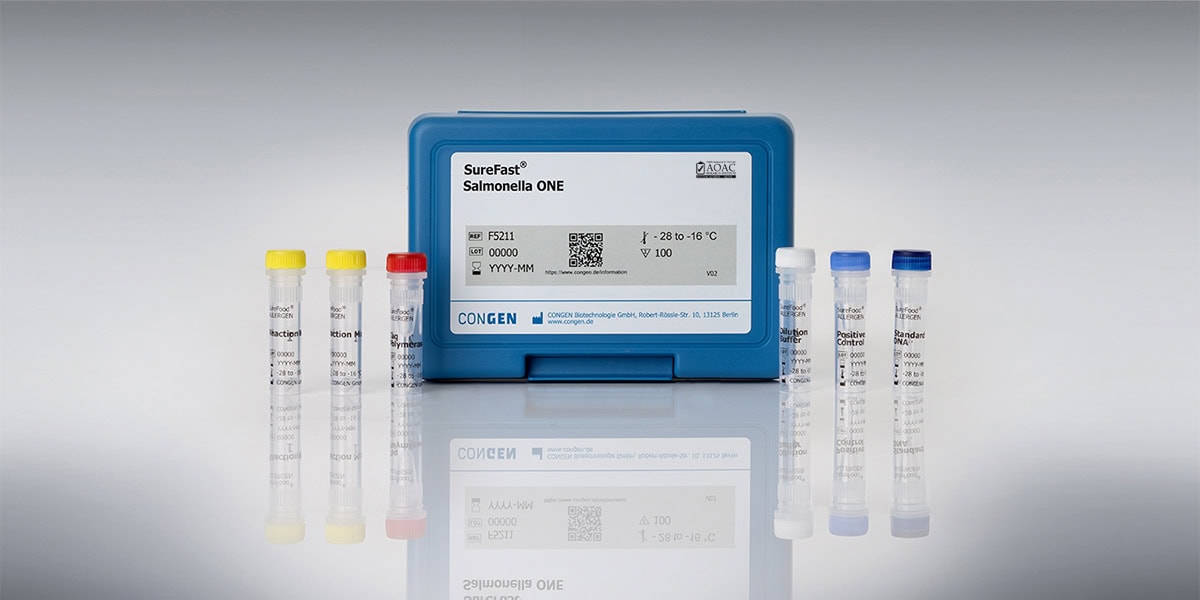
SureFast® Salmonella ONE
Art. No. F5211
Product info about SureFast® Salmonella ONE
Intended use
Salmonella spp. detection with real-time PCR
Two in one – Salmonella test kit with included DNA preparation
The kit is intended to be used for the fast and simple isolation and detection of Salmonella DNA from enrichment cultures. Each reaction contains an internal amplification control (IAC).
Benefits:
- MicroVal & AOAC approved
- Integrated DNA preparation
- Use the DNA directly for qPCR
- No centrifugation/automation needed
- Open system for all common qPCR thermocyclers
- FAM and VIC/HEX for internal amplification control
General Information
https://www.youtube.com/watch?v=cLrtwoYGTZcThe real-time PCR assay can be used with established real-time PCR instruments, equipped for detection of two fluorescence emissions in the FAM and VIC/HEX channel at the same time.
Internal validation was performed on Agilent Mx3005P, BioRad CFX 96, Roche LightCycler® 480 II, Roche cobas z 480 Analyzer, Roche LightCycler® 2.0, Applied Biosystems® 7500, Qiagen RotorGene Q, Cepheid SmartCycler®, Bio Molecular Systems MIC and LTF MyGo Pro.
External validation by MicroVal and AOAC was performed on Roche LightCycler® 480 II, Applied Biosystems® 7500, BioRad CFX 96, Bio Molecular Systems MIC and Agilent AriaMx.
Accessories
Dear customers,
we now provide the documents for our products in an electronic format which include the Instructions for Use (IFU), Safety Data Sheets (SDS), Application notes and the Certificate of Analysis (CoA). For batches placed on the market after 1st of January 2024, you can find our documents on the eIFU portal congen.de/information.
| Specifications | |
|---|---|
| Art. No | F5211 |
| Test format | 100 reactions |
| Sample preparation | Thermal lysis without additional steps |
| LOD (Detection Limit) | The SureFast® Salmonella ONE real-time PCR has a limit of detection of ≤ 5 DNA copies (1 cfu after pre-enrichment). The assay limit of detection depends on sample matrix, processing grade, DNA preparation and DNA content. |
| Files | |
|---|---|
| Certificates | MicroVal - Certificate No. 2014LR43 AOAC-RI - Certificate No. 081803 |
| Instructions | German/English |
| MSDS | Statement-MSDS-12-22-NG.pdf (English) |