RIDA®SMART BOX
Art. No. ZRSA-SB
Product info about RIDA®SMART BOX
Intended use
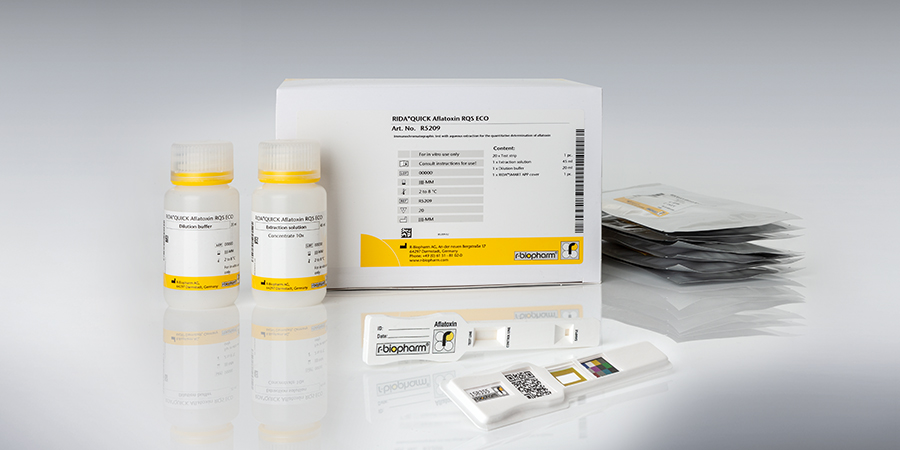
The RIDA®SMART BOX is an innovative benchtop lateral flow imaging unit designed for the automated readout of quantitative RIDA®QUICK LFD test strips for allergens and mycotoxins. In combination with the RIDA®SMART APP, the dedicated software application, the system enables precise analysis and quantification of relevant analytes. The device ensures reliable, standardized, and objective evaluation of test results, making it an essential tool for food and feed safety laboratories, quality control departments, and research institutions.
General Information
The RIDA®SMART BOX, in combination with the RIDA®SMART APP and R-Biopharm RIDA®QUICK Mycotoxin/Allergen LFD tests, provides the perfect system solution for your laboratory. Its robust design ensures optimal protection against dust while maintaining a stable and reliable measuring environment for the LFD test strips. The RIDA®SMART APP offers simple and intuitive operation, compatible with any* Android based system.
Advantages:
- Full flexibility: the RIDA®SMART BOX is not only still portable due to its small size, it can also be operated by means of a power bank via USB-C port.
- Compatibility: the RIDA®SMART BOX is usable with any Android device (Android 8 or higher).
State of the art connectivity – thanks to WiFi connectivity with the RIDA®SMART APP, results can be uploaded and exported via Email or to any cloud. - Continuous development process: state of the art system with features to simplify the workflow.
- Standardized measurement environment: the RIDA®SMART BOX improves the comparability of the precise and quantitative results obtained.
Accessories
Watch videoDear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.