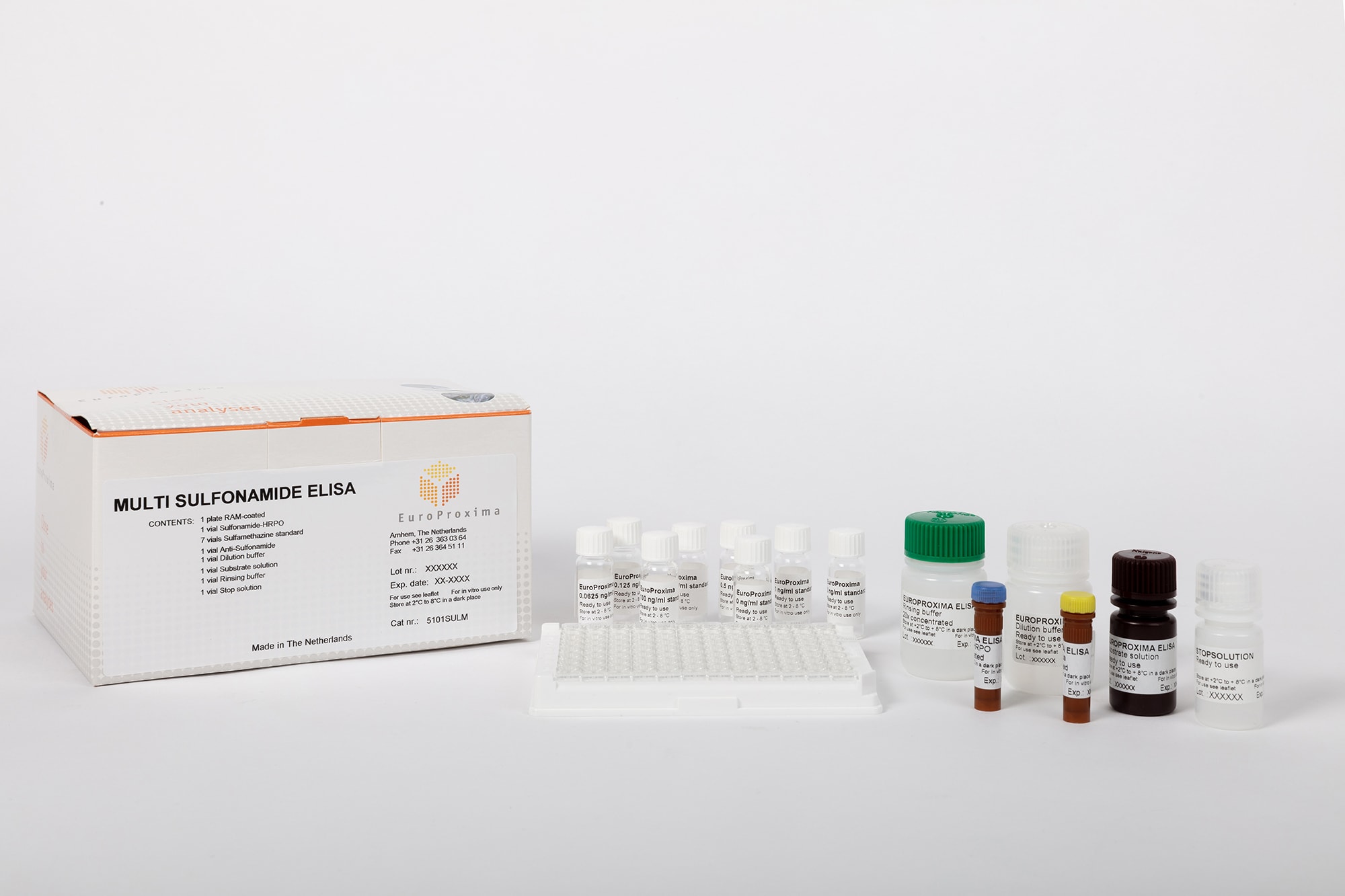
EuroProxima Multi-Sulfonamides
Art. No. 5101SULM
Product info about EuroProxima Multi-Sulfonamides
Intended use
EuroProxima Sulfonamides is a competitive enzyme immunoassay for quantitative analysis of a broad range of sulfonamides in urine, tissue, milk, egg, honey and shrimps.
General Information
Sulfonamides are a group of synthetic drugs whose molecules have a paminobenzenesulfonamide unit. They act as competitive antagonists of p-aminobenzoic acid, an important precursor of the essential folic acid for many bacteria and protozoa. Sulfonamides are antibiotics that are frequently used in veterinary and human medicine against bacteria and protozoa (coccidia).
Sulfonamides contained in food can lead to allergic or toxic reactions in consumers who are sensitive to these compounds. There is also a general concern that the widespread use of antibiotics could contribute to antibiotic resistance in pathogenic organisms. Normally, tissue residues in animals are controlled by removing the drug from the feed before slaughter. It is assumed that the sulfonamide concentrations then fall below the maximum residue level. However, due to contaminated feed or non-compliance with the withdrawal period, a number of animals arrive at slaughterhouses with a significantly excessive amount of medication in their tissue. The multisulfonamide ELISA can be used to detect residues in tissue, milk, honey, eggs and urine.
The following sulfonamides can be detected well below the maximum. The multisulfonamide ELISA can be used to detect residues in tissue, milk, honey, eggs and urine. The following sulfonamides can be detected well below the maximum residue level of 100 μg/kg set by the EC: Sulfamethazine, sulfadiazine, sulfamerazine, sulfisoxazole, sulfachloropyridazine and sulfachloropyrazine.
Dear customers,
we now provide the documents for our products in an electronic format which include the Instructions for Use (IFU), Safety Data Sheets (SDS), Application notes and the Certificate of Analysis (CoA). For batches placed on the market after 1st of January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/RBNL.
| Specifications | |
|---|---|
| Art. No | 5101SULM |
| Test format | Microtiter plate with 96 wells (12 strips with 8 individual wells each) |
| Incubation time | 90 min |
| LOD (Detection Limit) | Tissue (4 ng/ml), milk (<2.5 ng/ml), egg (3 ng/ml), honey (2 ng/ml), urine (5 ng/ml), shrimps (0.13 ng/ml) |
| Validated matrices | Tissue, milk, egg, honey, urine, shrimps |
| Evaluation | Microtiter plate photometer (450 nm) |
| Files | |
|---|---|
| Instructions | English |
| MSDS | |