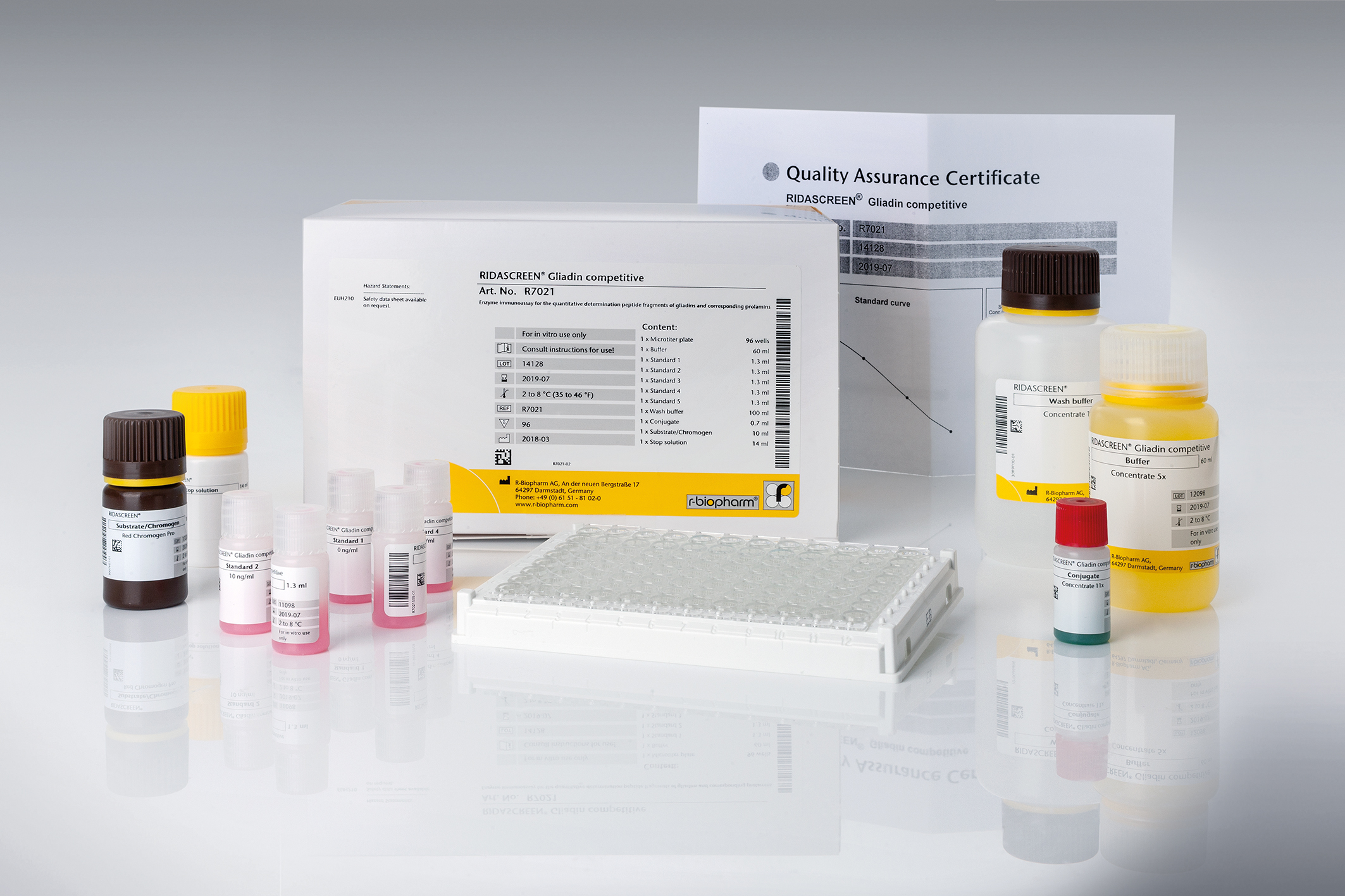
RIDASCREEN® Gliadin competitive
Art. No. R7021
Product info about RIDASCREEN® Gliadin competitive
Intended use
Specialty ELISA test method (competitive) for gluten detection in fermented and hydrolyzed food!
Ensures safe quantitative analysis of prolamine peptide fragments from wheat (gliadin), rye (secalin) and barley (hordein) in fermented or hydrolysed food, which are declared as « gluten-free ».
RIDASCREEN®Gliadin competitive is a R5-based competitive enzyme immunoassay for the quantitative analysis of prolamins from wheat (gliadin), rye (secalin) and barley (hordein) in fermented and hydrolyzed food. The used specific monoclonal antibody (R5) detects the amino acid sequences in prolamins that are toxic to coeliac disease patients. However, it does not react with epitopes of prolamins from oats which are tolerable for most celiac patients. Hence, it can be used to detect wheat, rye and barley gluten in oat products for gluten-free labelling purposes.
The R5 ELISA RIDASCREEN® Gliadin competitive is:
- validated AOAC official method of analysis (AOAC-OMA) Final Action Status (2015.05)
- official AACCI method (38-55.01) for beer, starch syrup and sourdough
- validated ASBC method (Beer-49)
Benefits:
- Quantifies intact prolamins and potential toxic fragments thereof (approx. 10 amino acids).
- Kit uses the R5 antibody recommended by the Codex and is the only commercially available competetive ELISA that uses the R5 antibody.
General Information
The use of gluten in foodstuffs is extremely common because of its useful effects on e.g. texture, moisture retention and flavor. However, gluten intolerance disorders like coeliac disease require a permanent gluten-free diet to avoid clinical symptoms. According to the Codex Alimentarius (CODEX STAN 118-1979) foods for special dietary use for persons intolerant to gluten may contain up to 20 mg/kg gluten to be tolerated by celiac patients. Foods containing < 20 mg/kg gluten can be labelled « gluten-free ».
The threshold of 20 mg/kg has been adopted by national legislations in many countries. Gluten of wheat, rye and barley is a mixture of prolamin and glutelin proteins. Prolamins from wheat are named gliadins. The prolamine content of wheat gluten is defined as 50 % (CODEX STAN 118-1979). R5-based ELISA methods, which have been calibrated against gliadin (prolamin part of wheat gluten), therefore need to be multiplied by a factor of 2 to calculate the gluten concentration.
During food processing like e.g. fermentation or hydrolysis, intact prolamin molecules are partly or completely degraded to small peptide fragments. For coeliac patients these fragments still remain dangerous even after digestion in the stomach. Single, small peptide sequences (motifs) cannot be detected by a sandwich ELISA format, because at least two epitopes are necessary for a sandwich ELISA. However, single peptide fragments can be detected with a competitive format as the RIDASCREEN® Gliadin competitive.
You can find further information on the scientific background of the test system in the following documents:
- AOECS (Association of European Coeliac Societies) recommends the R5 ELISA (competitive ELISA) for hydrolyzed gluten. View more
- AACI. View more
- A comparison of gluten levels in labeled gluten-free and certified gluten-free foods sold in the United States (T. Thompson, S. Simpson) published in European Journal of Clinical Nutrition (2014)
Following extraction solutions are validated for this method:
- 60 % ethanol
Accessories
Videos
Vous êtes actuellement en train de consulter le contenu d'un espace réservé de YouTube. Pour accéder au contenu réel, cliquez sur le bouton ci-dessous. Veuillez noter que ce faisant, des données seront partagées avec des providers tiers.
Plus d'informationsDear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | R7021 |
| Test format | Microtiter plate with 96 wells (12 strips with 8 removable wells each) |
| Sample preparation | Homogenization and extraction |
| Incubation time | 40 min, room temperature |
| LOD (Detection Limit) | 2.3 mg / kg (ppm) Gliadin (mean) or to 4.6 mg / kg (ppm) Gluten 1.9 - 2.6 mg / kg (ppm) Gliadin* *depending on matrix |
| LOQ (Limit of quantification | 5 mg / kg (ppm) Gliadin or 10 mg / kg (ppm) Gluten |
| Validated matrices | Gluten-free beer, rice beer, maize flour, sourdough, sugar beet syrup |
| Detected analyte | • The main epitope of the R5 antibody is the amino acid sequence QQPFP which is part of many celiac-toxic sequences. |
| Available application notes | • Analysis of gluten in supplemental enzymes. • Improving quantification range to 2.5 mg / kg (ppm) gliadin. |
| Evaluation | Microtiter plate spectrophotometer (450 nm) |