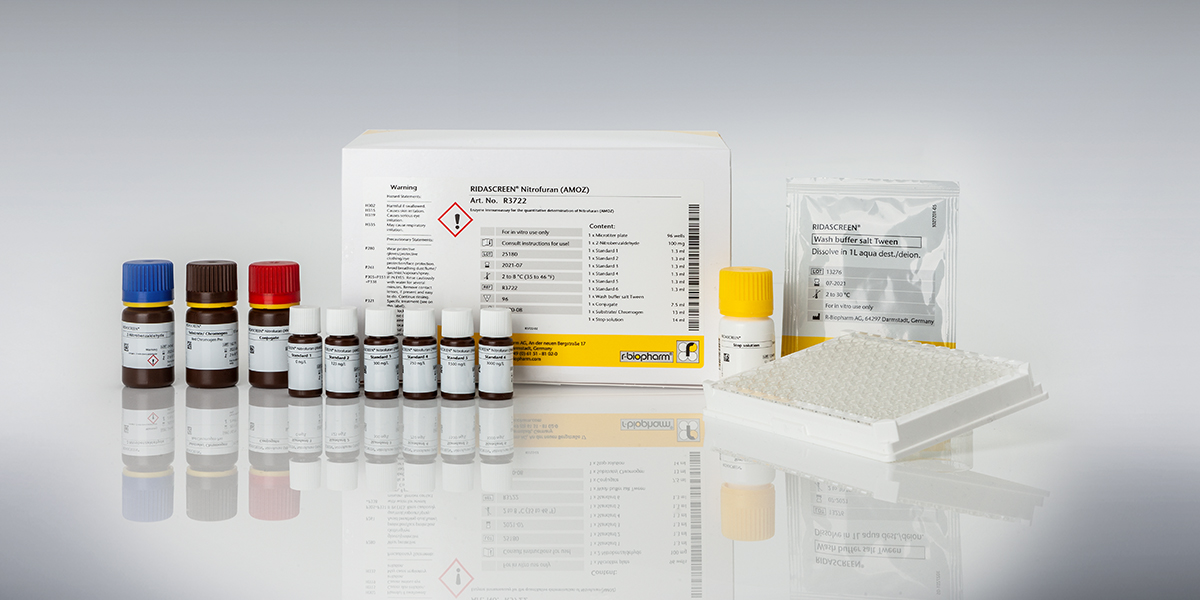
RIDASCREEN® Nitrofuran (AMOZ)
Art. No. R3722
Product info about RIDASCREEN® Nitrofuran (AMOZ)
Intended use
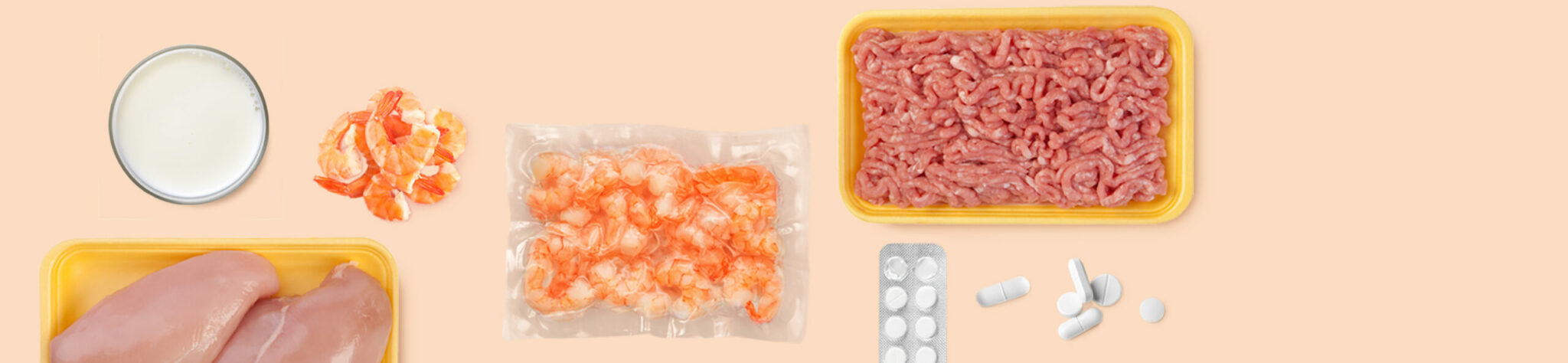
RIDASCREEN® Nitrofuran (AMOZ) is a competitive enzyme immunoassay for the quantitative determination of AMOZ in shrimp, fish, meat and poultry.
General Information
Nitrofurans are synthetic broad-spectrum antibiotics, which are frequently used in animal production due to their excellent antibacterial and pharmacokinetic properties. They have also been used as growth promoters during the production of shrimp, poultry and pigs. Long term animal experiments have shown that the parent compounds and their metabolites have carcinogenic and mutagenic characteristics. This led to the prohibition of nitrofurans for the treatment of animals used for food production. In 1993, the EU banned the nitrofurans furaltadone, nitrofurantoin and nitrofurazone for use in animals used as sources of food, and in 1995 the use of furazolidone was also prohibited. The analysis of nitrofurans are based on the detection of the tissue bound metabolites of nitrofurans. The parent compounds are difficult to detect accurately since they are metabolized very rapidly after treatment. The tissue bound nitrofuran metabolites however are present for a long time after administration and they are used to detect nitrofuran abuse.
Parent compound / Metabolite / Abbreviation
Nitrofurantoin / 1-Aminohydantoin / AHD
Furaltadone / 3-Amino-5-morpholinomethyl-2-oxazolidinone / AMOZ
Furazolidone / 3-Amino-2-oxazolidinone / AOZ
Nitrofurazone / Semicarbazide / SEM
Prior to analysis, the metabolites have to be derivatized by incubation with 2-Nitrobenzaldehyde into NP-AHD, NP-AMOZ, NP-AOZ and NP-SEM.
Benefits:
- One sample preparation for the nitrofurans AHD, AMOZ, AOZ and SEM as well as for chloramphenicol.
- High sensitivity – allows compliance with European legislation (MRPL value: 1000 ng/kg (ppt) – Commission Decision 2003/181/EC).
- Assay results within 45 minutes available.
Dear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | R3722 |
| Test format | Microtiter plate with 96 wells (12 strips with 8 wells each) |
| Sample preparation | Homogenization, derivatization, extraction, centrifugation, evaporation, defattening. Depending on the workflow in the laboratory, a 3-hour or an over-night derivatization may be performed. For highest precision, the over-night method is recommended. All specifications in this manual were determined using this sample preparation; details on the 3 hour method are published in the validation report. |
| Incubation time | 45 min |
| LOD (Detection Limit) | Shrimp approx. 30 ng/kg, fish approx. 40 ng/kg, meat (bovine) approx. 40 ng/kg, meat (porcine) approx. 65 ng/kg, poultry (chicken, turkey) approx. 40 ng/kg |
| Detection Capability | 300 ng/kg for all matrices |
| Validated matrices | Shrimp, fish, meat (bovine, porcine), poultry (chicken, turkey). |
| Detected analyte | Nitrofuran (AMOZ) in shrimp, fish, meat and poultry. |
| Available application notes | Fish and shrimp. |
| Evaluation | Microtiter plate spectrophotometer (450 nm) |