Recent news in Food & Feed Analysis
- Home
- /
- New gluten test kits...
New gluten test kits for a growing gluten free food market

The gluten free market is experiencing unprecedented growth, with a global valuation of $6.45 billion and a compound annual growth rate (CAGR) of 9.8%, according to Grand View Research. This surge is driven by increasing consumer awareness of celiac disease and gluten sensitivity, as well as a broader trend toward health-conscious eating. However, as demand escalates, food and beverage manufacturers face significant challenges in ensuring their products meet stringent gluten-free standards. R-Biopharm introduces a suite of gluten test kits and data management to meet these challenges.
Challenges in Gluten Free Food Production
Producing gluten-free foods involves meticulous attention to every stage of the production process to prevent contamination. Key challenges include:
- Sourcing Gluten-Free Ingredients: Ensuring raw materials are free from gluten contamination during cultivation, harvest, and storage.
- Preventing Cross-Contamination: Avoiding gluten exposure during transportation, storage, and processing, especially when facilities handle both gluten-containing and gluten-free products.
- Accurate Labeling: Maintaining precise labeling to prevent consumer misinformation and potential health risks.
Addressing these challenges is crucial for consumer safety and maintaining brand integrity.
Integrated Gluten Management: A Comprehensive Solution for Gluten Testing
To assist manufacturers in navigating these complexities, R-Biopharm offers an Integrated Gluten Management solution. This approach provides a suite of gluten test kits designed to monitor gluten levels throughout the entire production chain, ensuring compliance with gluten free standards.
Key Components of Integrated Gluten Management
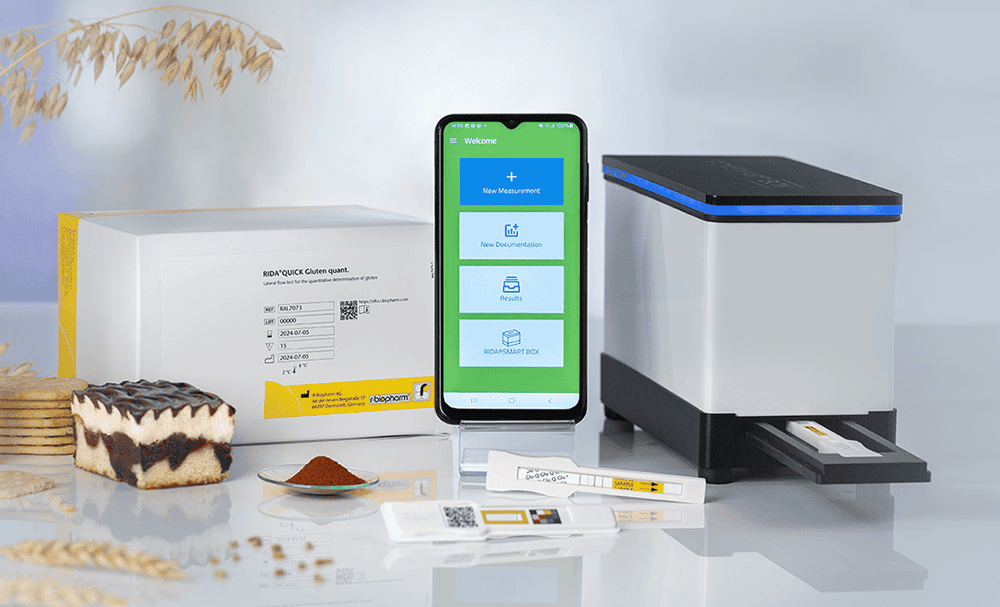
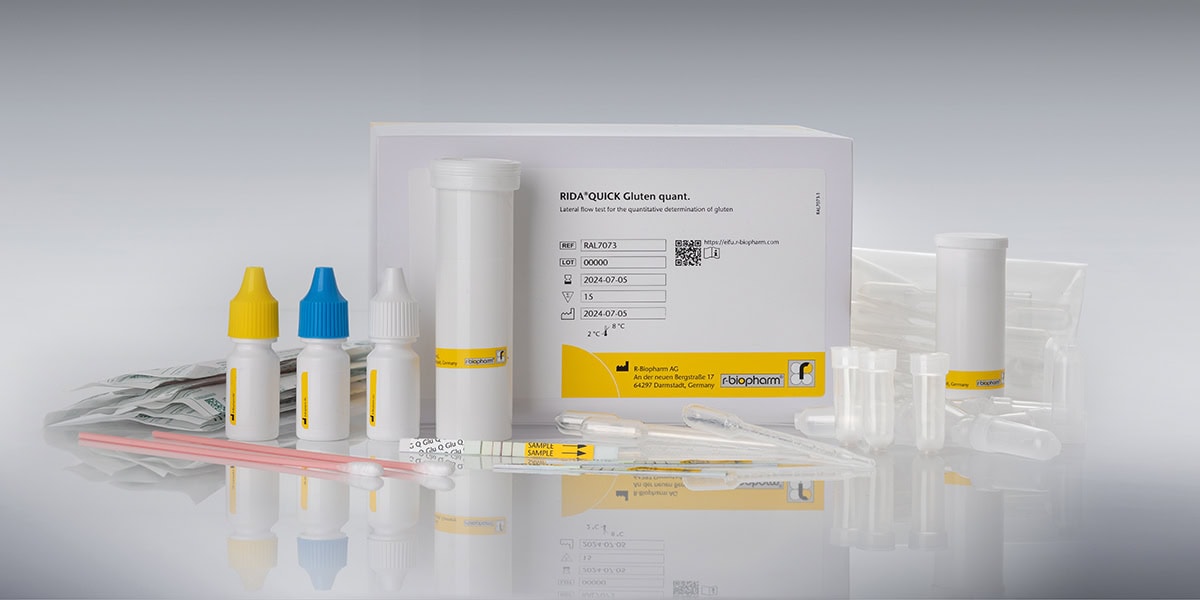
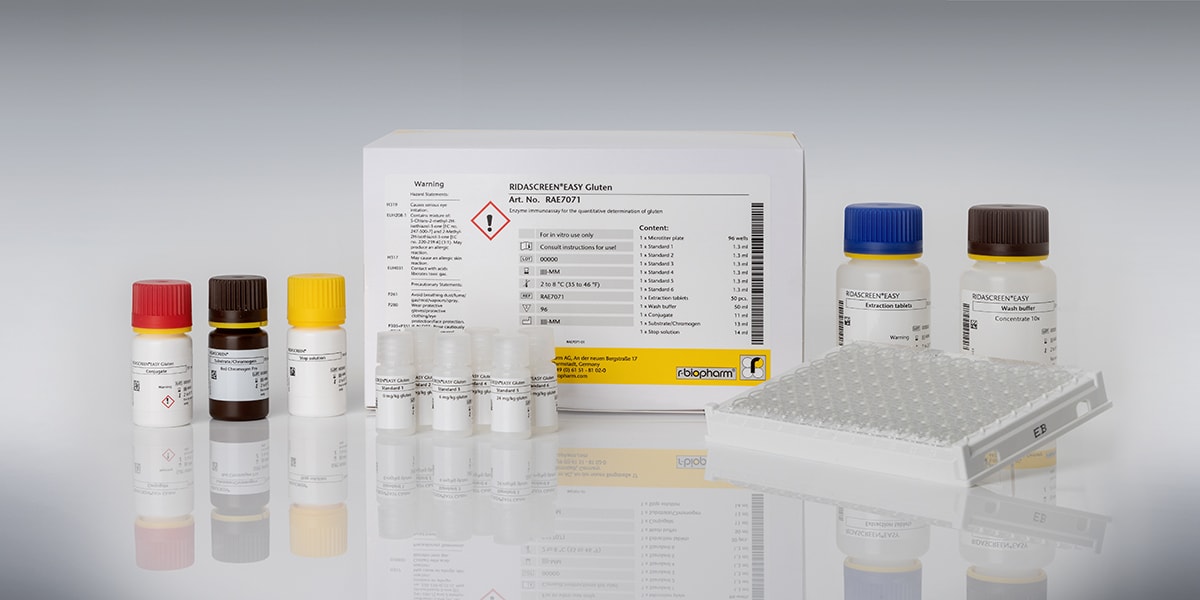
- Raw Material Testing: Using gluten test strips, like the RIDA®QUICK Gluten quant, manufacturers can rapidly and quantitatively assess gluten presence in raw materials on-site. This lateral flow test is ideal for quick decision-making and gluten detection during the intake of raw materials. Alternatively, samples of incoming goods can be tested with an ELISA-based method, like the RIDASCREEN®EASY Gluten.
- Process Monitoring: Regular testing of production surfaces and equipment is vital to prevent cross-contamination. Our gluten test strips, like the RIDA®QUICK Gliadin test kit enable rapid detection of gluten residues on surfaces, ensuring that cleaning protocols are effective. In-process samples can be tested in the laboratory by ELISA as well.
- Finished Product Testing: The RIDASCREEN® Gliadin ELISA allows precise quantification of gluten, also in processed foods. This is essential for in-house laboratory analyses to verify that gluten levels remain within acceptable limits throughout production. The RIDASCREEN® Gliadin in combination with the Cocktail is Codex Alimentarius type I method (CODEX STAN 234-1999) and AOAC Official Method of Analysis (OMA 2012.01).
- Data Management: Integrating test results into a centralized system like the RIDA® Universe allows for comprehensive monitoring and traceability. This facilitates compliance reporting and continuous improvement in gluten management practices.
Benefits of Implementing R-Biopharm’s Integrated Gluten Management
- Enhanced Product Safety: Regular testing at critical control points minimizes the risk of gluten contamination, safeguarding consumer health.
- Regulatory Compliance: Accurate monitoring ensures adherence to global gluten-free labeling standards, such as the European Union’s Regulation (EU) 828/2014 and the U.S. FDA’s gluten-free labeling rule.
- Meeting Industry Standards: Gluten-free certification by organizations like the Gluten-Free Certification Organization (GFCO) or the Association of European Coeliac Societies (AOECS) stipulate specific requirements for analytical methods used for quality assurance.
- Consumer Trust: Demonstrating a commitment to rigorous gluten management fosters brand loyalty among consumers seeking safe gluten-free options.
Conclusion
As the gluten-free market continues to expand, manufacturers must proactively address the challenges associated with gluten contamination. Implementing R-Biopharm’s Integrated Gluten Management solution provides a robust framework for ensuring product safety, regulatory compliance, and consumer satisfaction. By leveraging advanced gluten test kits and comprehensive monitoring strategies, producers can confidently meet the rising demand for high-quality gluten-free foods.
For more information on Integrated Gluten Management and to explore suitable gluten test kits for your production process, visit R-Biopharm’s Integrated Gluten Management page.
Integrated gluten management
Producing gluten-free foods involves several critical steps to ensure a safe product for consumers with celiac disease or gluten sensitivity. Compliance with gluten-free labeling legislations or certifications is essential. From raw materials and ingredients to transport, production, storage and the final product, the challenge is to produce a healthy, nutritional product that meets consumers’ expectations and prevents gluten contamination at every step of the production chain.
Read more