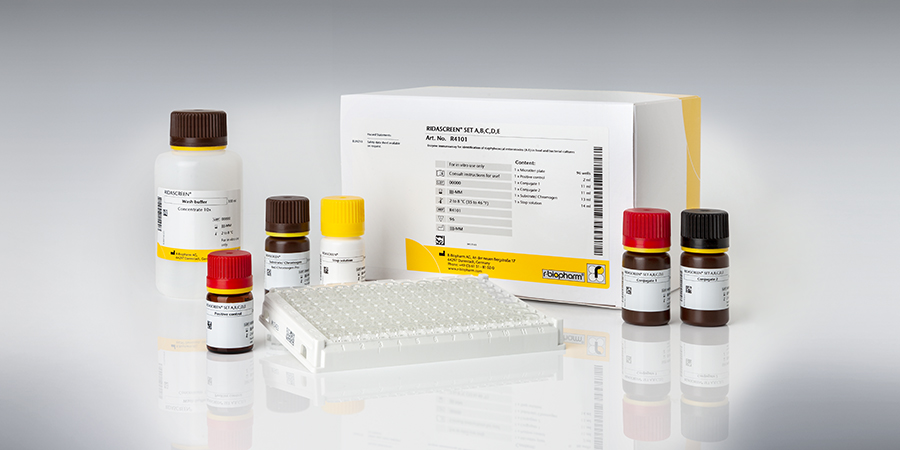
RIDASCREEN® SET A,B,C,D,E
Art. No. R4101
Product info about RIDASCREEN® SET A,B,C,D,E
Intended use
RIDASCREEN® SET A,B,C,D,E is a sandwich enzyme immunoassay for the identification of Staphylococcus enterotoxins A, B, C, D and E in fluid and solid foods as well as in bacterial cultures. Based on its sensitivity the RIDASCREEN® SET A,B,C,D,E test is consequently clear superior to the immunodiffusion procedure which has a detection limit of 0.1 mg / ml.
General Information
Staphylococci belong to the family of micrococci. Staphylococcus aureus, Staphylococcus hyicus as well as Staphylococcus intermedius produce one or more heat stable proteins which behave as enterotoxins (SET). SETs are the main causative agents of food poisoning next to Salmonellosis and Campylobacteriosis. Generally, it is assumed that a population of 5 x 105 cells of enterotoxin-producing S. aureus strains per gram of food is required to lead to an intoxication. However, other studies show that only 100 – 200 ng of Staphylococcus enterotoxins can lead to symptoms of food poisoning. SET intoxications have been frequently associated with pasta, finished meat products, ham, pies, chicken meat products, fish, fish products, milk, milk products, ice-cream, egg products, salads, pastries and cake stuffing as well as preparations from these food products. The enterotoxins of the serological group A, B, C, D, E are very significant.
Dear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | R4101 |
| Test format | Microtiter plate with 96 wells (12 strips with 8 wells each), one strip is necessary for analysis of one sample, 12 tests in total. |
| Sample preparation | „Official European Preparation Method“ (dialysis concentration method) or simplified method. |
| Incubation time | 2 h 45 min |
| LOD (Detection Limit) | Simplified working up: - Liquid samples: 0.25 ng toxin / ml sample, - Solid samples: 0.375 ng toxin / g sample, - Supernatants from bacterial cultures: 0.25 ng toxin / ml sample. Dialysis concentration: - Liquid samples: 0.05 ng toxin / ml sample, - Solid samples: 0.05 ng toxin / g sample. |
| Cross Reactivity. | Crossreactivities are known to occur between antibody / toxin: The percentage of crossreactivities is between 10 – 20 %. Some food matrices (e.g.: milk, sausages) may enhance or weaken the crossreactivity. |
| Detected analyte | Staphylococcus enterotoxins A, B, C, D, E |
| Evaluation | Microtiter plate spectrophotometer |