Recent news in Food & Feed Analysis
- Home
- /
- Reliable, Efficient, Certified: Achieving...
Reliable, Efficient, Certified: Achieving AOAC certification with 11+Myco MS-PREP®
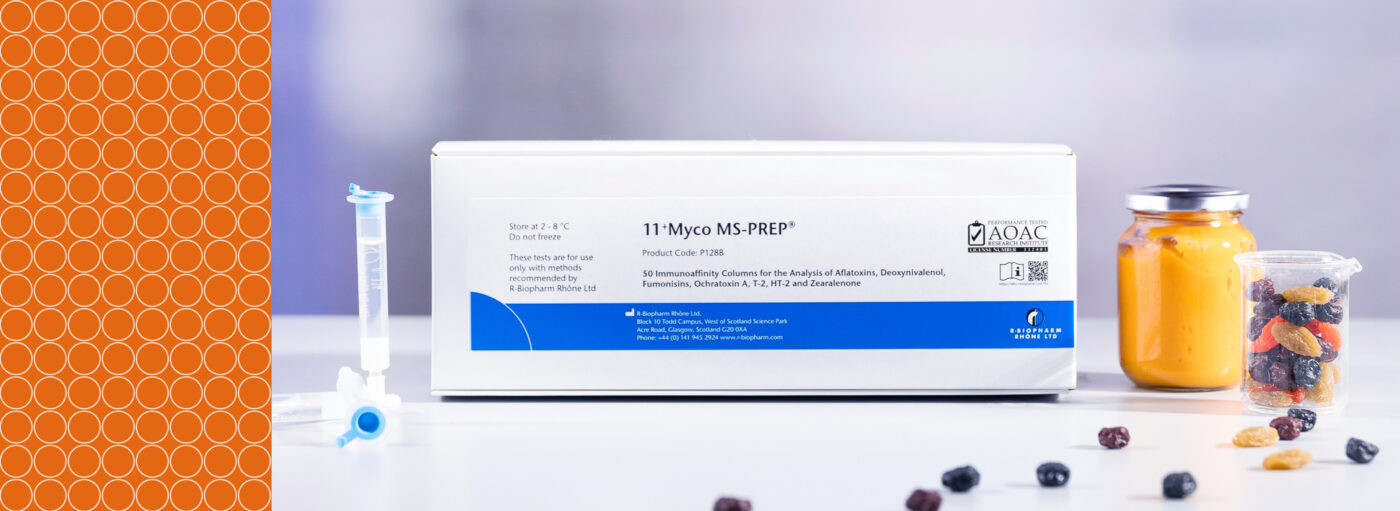
What is AOAC PTM certification?
AOAC Performance Tested Methods (PTM) certification is a recognised standard that verifies product methods through comprehensive laboratory testing. This certification ensures that methods meet the highest of standards.
In the case of R-Biopharm Rhône’s 11+Myco MS-PREP® columns, a single multi-mycotoxin extraction method for multi-mycotoxin analysis of cereals, baby food, spices, and animal feed has been tested by LC-MS/MS and granted AOAC RI-112401 PTM certification. Achieving AOAC PTM certification is a significant milestone, providing customers with confidence in the robustness and performance of the method.
Working towards achieving certification
R-Biopharm Rhône’s journey to 11+Myco MS-PREP® PTM method approval began several years ago, with a plan to develop a comprehensive analytical method capable of addressing the challenges faced by analysts to quantify multiple analytes in a variety of matrices using a single extraction method.
The difficulty was to develop a single extraction method for the detection of 11 toxins, each with their own unique characteristics, while ensuring the method met the required regulatory specifications including LOQ (Limit of Quantification) across these variations. It was no small feat to find a single extraction solution capable of producing acceptable recoveries for all toxins, particularly when naturally occurring mycotoxins were present, further complicating detection. Fast forward to late 2024 after many hours in the lab and gathering data, R-Biopharm Rhône managed to overcome these challenges and our method was published and received AOAC certification (AOAC RI-112401) for the determination of Aflatoxins, Fumonisins, Deoxynivalenol, Ochratoxin A, Zearalenone, HT-2, and T-2 Toxins in Cereals, Baby Food, Spices, and Animal Feed.
What does this mean for customers?
Gaining AOAC PTM certification means that customers can trust our 11+Myco MS-PREP® method to deliver consistent, high-quality results that comply with legislation. By using a single extraction approach, laboratories can significantly streamline their workflows, reducing time and resources without losing performance.
The project has not only strengthened R&D capabilities but also gained significant recognition within the scientific community, featuring in the “most read” section in the Journal of AOAC. The approval has been an outstanding accomplishment and reflects the expertise and dedication of the team at R-Biopharm Rhône.