Recent news in Food & Feed Analysis
- Home
- /
- AOAC OMA First Action...
AOAC OMA First Action Approval for SureFast® Listeria 3plex ONE

We are proud to announce that our SureFast® Listeria 3plex ONE multiplex real-time PCR kit has received AOAC Official Method of Analysis (OMA) approval, a globally recognized standard for method validation.
This includes the SureFast® Listeria 3plex ONE Kit and the SureFast® PREP Bacteria Kit in a Variety of Foods (including dairy, meat, seafood, produce, and other processed foods) and Environmental Samples: Multi-Laboratory Validation, First Action 2025.04
This milestone underscores Congen’s and R-Biopharm’s commitment to providing regulatory-trusted, efficient, and user-friendly solutions for the food safety industry.
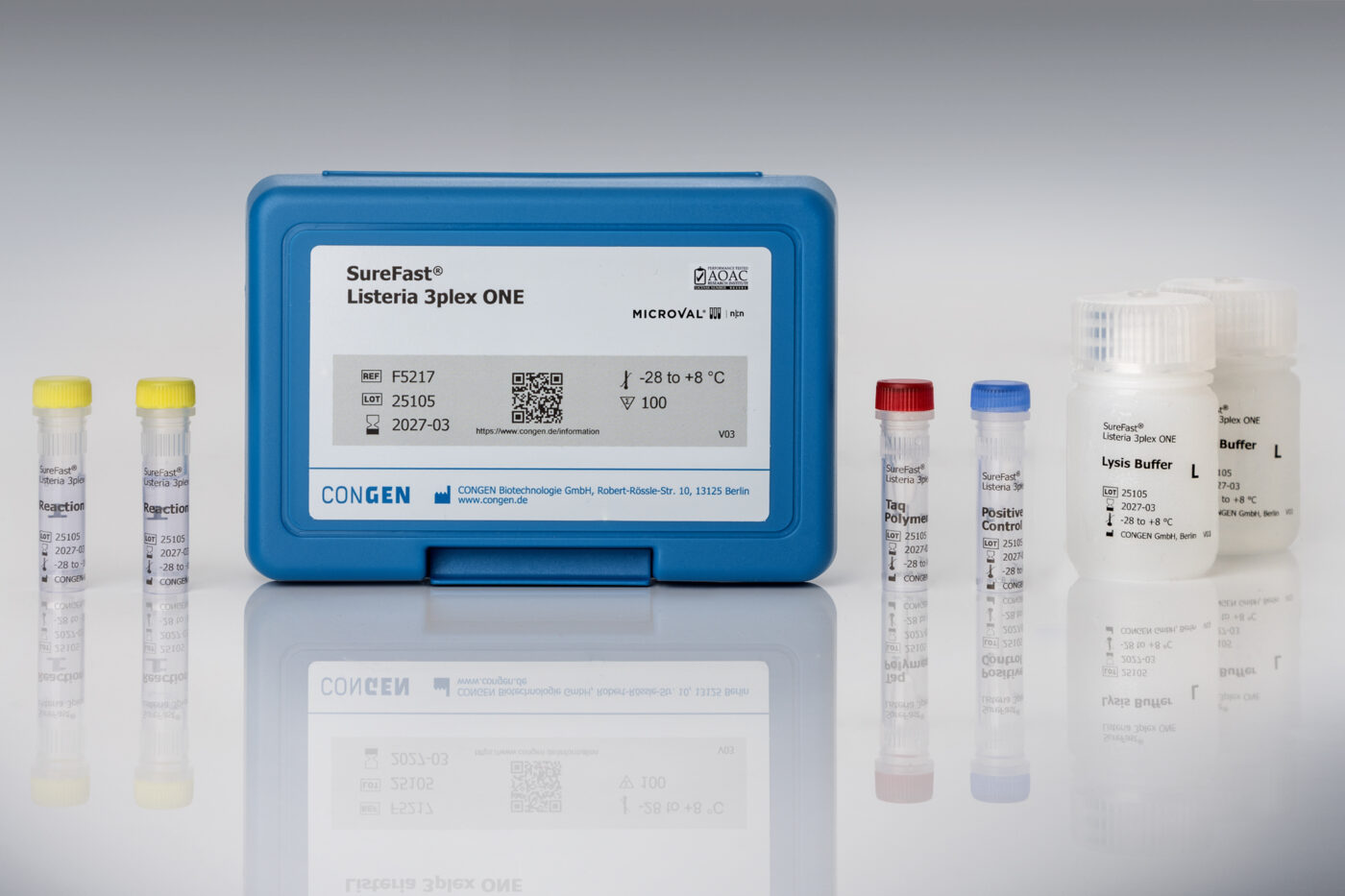
Two-in-One: SureFast® Listeria 3plex ONE qPCR test kit including DNA preparation
Key Benefits:
- Simple and time-saving DNA preparation
- Direct use of DNA for qPCR after lysis
- Simultaneous detection of Listeria spp. & Listeria monocytogenes
- Open system compatible with standard qPCR thermocyclers (HEX, ROX Cy5)
- Simultaneous analysis with other SureFast® Microbiology qPCR Kits
Key benefits for laboratories and food producers include:
- Regulatory confidence: AOAC OMA approval is widely recognized by auditors and accreditation bodies worldwide.
- Streamlined workflow: The included DNA prep step reduces handling, eliminates the need for separate extraction kits, and minimizes potential errors.
- Broad applicability: Validated across multiple food types, ensuring reliability for diverse production environments.
- Multiplex efficiency: Detects Listeria monocytogenes and Listeria spp. in a single assay, saving time, resources, and labor.
For more information, visit https://food.r-biopharm.com/products/surefast-listeria-3plex-one/