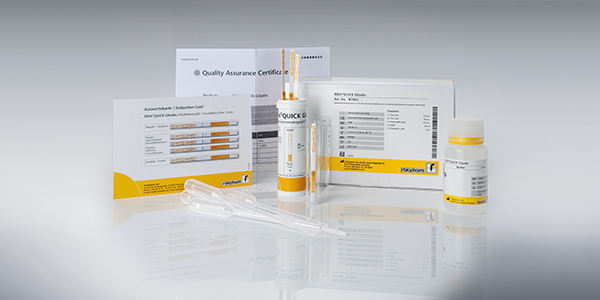
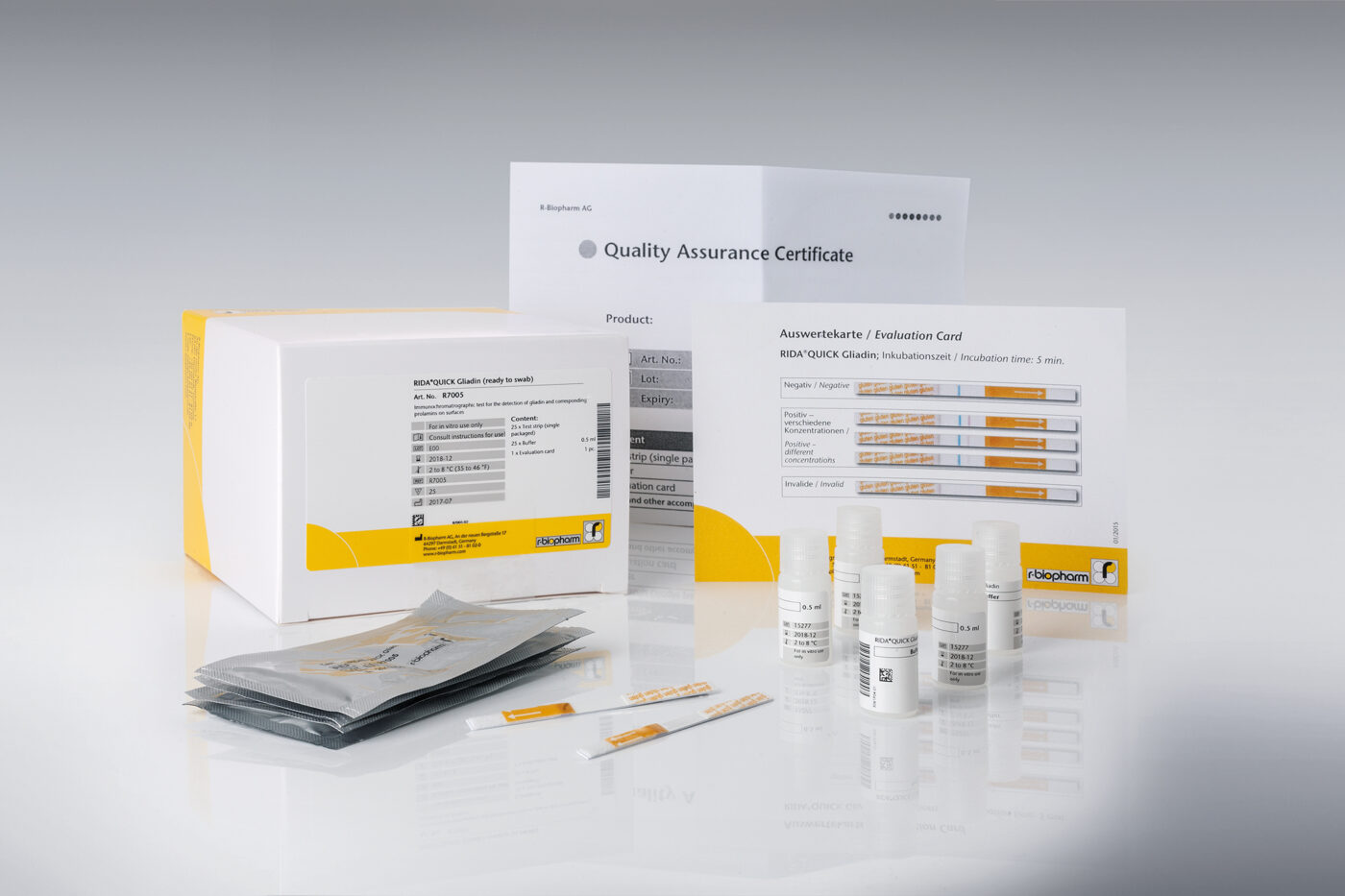
RIDA®QUICK Gliadin
Art. No. R7003
Product info about RIDA®QUICK Gliadin
Intended use
Fast and simple qualitative LFD test method for the detection of gluten!
Ensures safe, fast and simple qualitative analysis of gluten on surfaces, in clean-in-pace (CIP) water and food (raw and processed).
RIDA®QUICK Gliadin is an R5-based immunochromatographic test for the qualitative determination of gluten-contamination. The test has been developed for the detection of low amounts of gluten (contamination). No high-dose-hook-effect is observed at high concentrations.
The RIDA®QUICK Gliadin has the following international certificates/approvals:
- AOAC-RI: PTM certification no. 101702 (for swabbing and cleansing waters)
- AOAC-OMA (2015.16) for corn-based food matrices using ethanol or Cocktail (patented) extraction
- AACCI – international approved method 38-60.01
Benefits:
- Only qualitative immunochromatographic lateral flow test using the R5 antibody on the market
- Test strip can directly be used for surface swabbing (no external swab needed)
- Easy documentation by use of the RIDA®SMART APP Allergen (available in the Google Play Store) and availability of cloud solution
We offer three different kit package variants, all containing the same test strip:
- RIDA®QUICK Gliadin (Art. No. R7003) – 25 strips in reclosable tube, 25 plastic pipettes, 30 vials, one buffer bottle
- RIDA®QUICK Gliadin (single packaged) (Art. No. R7004) – 25 strips single packaged, 30 vials, one buffer bottle
- RIDA®QUICK Gliadin (ready to swab) (Art. No. R7005) – 25 strips single packaged, 25 vials pre-filled with buffer
General Information
The use of gluten in foodstuffs is extremely common because of its useful effects on e.g. texture, moisture retention and flavor. However, gluten intolerance disorders like coeliac disease require a permanent gluten-free diet to avoid clinical symptoms. According to the Codex Alimentarius (CODEX STAN 118-1979) foods for special dietary use for persons intolerant to gluten may contain up to 20 mg/kg gluten to be tolerated by celiac patients. Foods containing < 20 mg/kg gluten can be labelled “gluten-free”.
The threshold of 20 mg/kg has been adopted by national legislations in many countries. Gluten of wheat, rye and barley is a mixture of prolamin and glutelin proteins. Prolamins from wheat are named gliadins. The prolamine content of wheat gluten is defined as 50 % (CODEX STAN 118-1979).
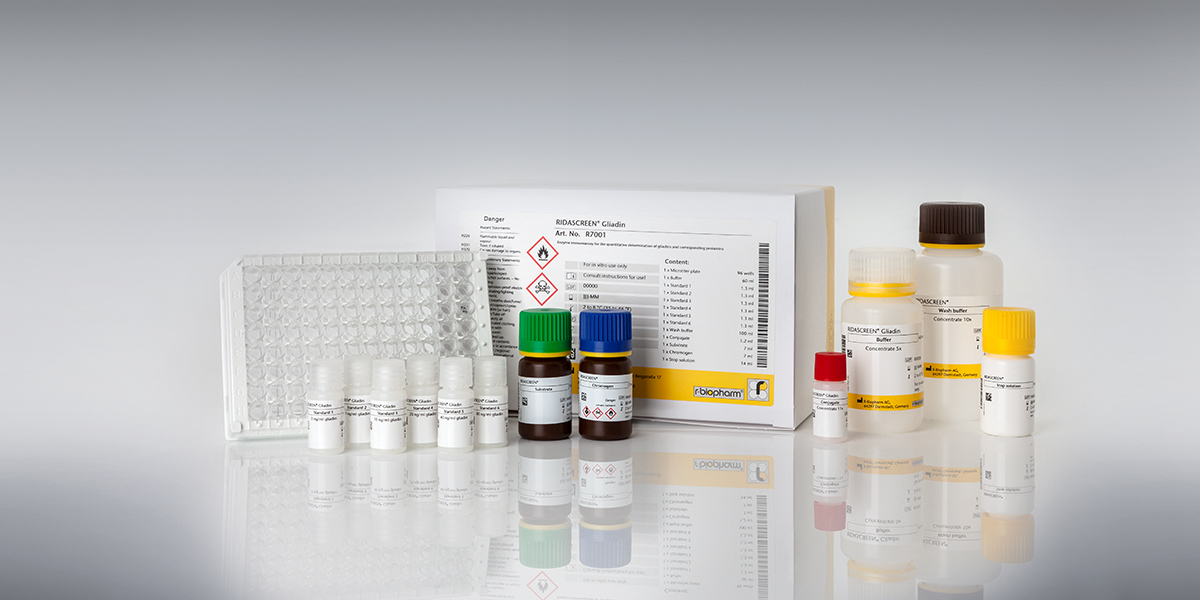
The official type I method for gluten determination of the Codex Alimentarius is an ELISA, which uses the R5 antibody in combination with a special extraction buffer, the Cocktail (patented) (Mendéz method). The RIDASCREEN® Gliadin ELISA (Art. No. R7001) fulfills this requirement. The RIDA®QUICK Gliadin test strips also use the R5 antibody and show a good correlation with the official method R5 ELISA RIDASCREEN® Gliadin. Only R-Biopharm AG is authorized to use the R5 antibody in immunochromatographic lateral flow tests.
You can find further information on the scientific background of the test system in the following documents:
- Validation of a qualitative R5 dip-stick for gluten detection with a new mathematical-statistical approach. View article
- Determination of Gluten in Processed and Nonprocessed Corn Products by Qualitative R5 Immunochromatographic Dipstick: Collaborative Study, First Action 2015.16. View article
- The Validation of the RIDA®QUICK Gliadin for AOAC Research Institute. View article
Following extraction solutions are validated for this method:
- Cocktail (patented) (Art. No. R7006 / R7016)
- RIDA® Cocktail ECO (Art. No. R7080) *
- 60% Ethanol
Additional extraction solutions are:
- RIDA® Extraction Solution (colorless) (Art. No. 7098)
Accessories
- Cocktail (patented)
- Cocktail ECO
- RIDA® Extraction Solution (colorless)
- Set of 3 processed Gliadin Assay Controls
- RIDA®SMART APP Allergen
Videos
You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationYou are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationDear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | R7003 |
| Test format | 25 x test strips |
| Incubation time | 5 min, RT |
| LOD (Detection Limit) | • Surfaces: approx. 1.6 - 3 µg gluten / 100 cm2 • CIP water (w/o cleansing agent): approx. 10 ng/mL gluten • CIP water (with cleansing agent): approx. 50 - 100 ng/mL gluten • Raw material (ethanolic extraction): approx. 4.4 mg/kg gluten • Processed food samples (Cocktail (patented) extraction: approx 6.3 mg/kg gluten |
| Validated matrices | • Raw and processed food samples (e.g. corn flour, snack, cookies, rice flour, soy flour, sausage) |
| Detected analyte | • The main epitope of the R5 antibody is the amino acid sequence QQPFP which is part of many celiac-toxic sequences. |
| Available application notes | • Sample preparation for processed food with the RIDA® Extraction Solution • Sample preparation for polyphenol containing raw material using fish gelatine. |
| Evaluation | Visual evaluation |