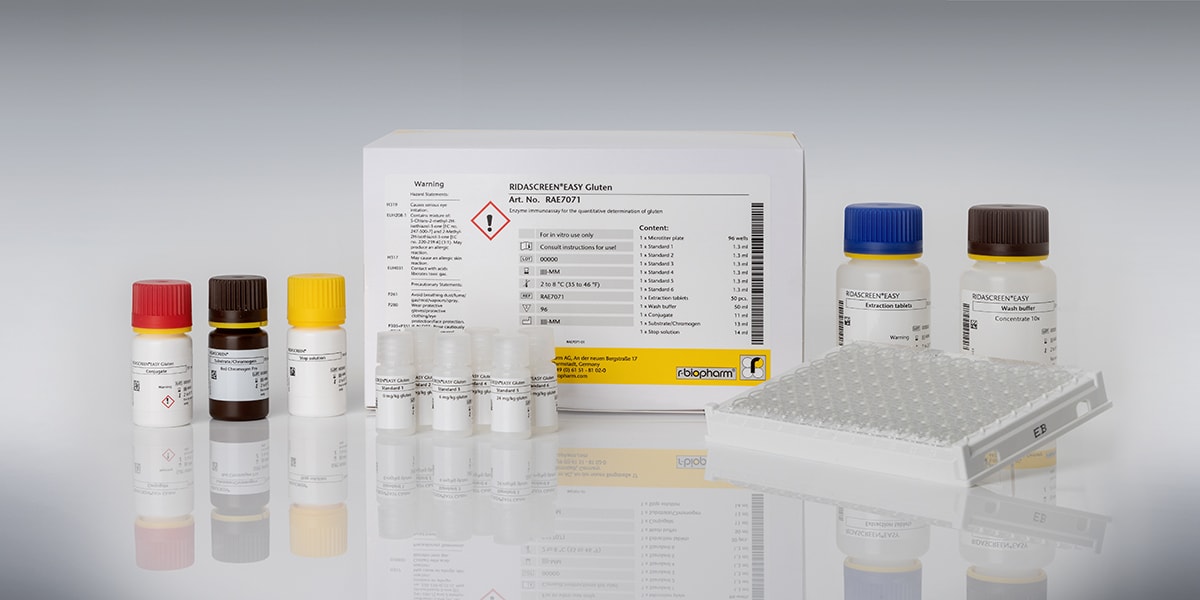
RIDASCREEN®EASY Gluten
Art. No. RAE7071
Product info about RIDASCREEN®EASY Gluten
Intended use
Fast and easy ELISA test method for gluten detection!
Ensures a safe, fast and easy quantitative analysis of gluten residues from wheat, rye and barley within 30 minutes, delivering results that are in line with the Codex Alimentarius Type I Method (see RIDASCREEN® Gliadin, Art. No. R7001).
RIDASCREEN®EASY Gluten is an R5-based sandwich enzyme immunoassay for the quantitative analysis of contaminations by gluten from wheat, rye and barley in food. The used specific monoclonal antibody (R5) detects the amino acid sequences in the prolamin fraction of gluten that are toxic to coeliac disease patients. However, it does not react with epitopes of prolamins from oats which are tolerable for most celiac patients.
Benefits:
- Simple extraction with the EASY extraction tablet
- Results expressed as gluten (no conversion factor needed)
- Enhanced validation: usage of incurred matrices as well as wheat, rye and barley as contaminant – meeting the latest AOAC guidelines
General Information
The use of gluten in foodstuffs is extremely common because of its useful effects on e.g. texture, moisture retention and flavor. However, gluten intolerance disorders like coeliac disease require a permanent gluten-free diet to avoid clinical symptoms. According to the Codex Alimentarius (CODEX STAN 118-1979) foods for special dietary use for persons intolerant to gluten may contain up to 20 mg/kg gluten to be tolerated by celiac patients. Foods containing < 20 mg/kg gluten can be labelled “gluten-free”.
The threshold of 20 mg/kg has been adopted by national legislations in many countries. Gluten of wheat, rye and barley is a mixture of prolamin and glutelin proteins. Prolamins from wheat are named gliadins. The prolamine content of wheat gluten is defined as 50 % (CODEX STAN 118-1979). R5-based ELISA methods, which have been calibrated against gliadin (prolamin part of wheat gluten), therefore need to be multiplied by factor of 2 to calculate the gluten concentration. Due to the calibration against gluten, the RIDASCREEN®EASY Gluten assay provides results directly in mg/kg gluten. Thus, the calculation factor is not needed any longer.
Following extraction solutions are validated for this method:
- RIDASCREEN®EASY Extraction Tablets (Art. No. RAA0008)*
Accessories
Dear customers,
we provide the documents for our products in an electronic format which include the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01. January 2024, you can find our documents on the eIFU portal eifu.r-biopharm.com/food.
| Specifications | |
|---|---|
| Art. No | RAE7071 |
| Test format | Microtiter plate with 96 wells (12 strips with 8 removable wells each) |
| Sample preparation | Homogenization and extraction |
| Incubation time | 30 min, room temperature |
| LOD (Detection Limit) | 0.8 mg/kg (ppm) gluten (average of wheat, rye, barley) depending on matrix |
| LOQ (Limit of quantification | 3 mg/kg (ppm) gluten (average of wheat, rye, barley) |
| Cross Reactivity. | No cross-reaction was detected in 108 tested foods. |
| Validated matrices | Baked goods, sauces, chocolate containing desserts, spices |
| Detected analyte | • The main epitope of the R5 antibody is the amino acid sequence QQPFP which is part of many celiac-toxic sequences. |
| Evaluation | Microtiter plate spectrophotometer (450 nm) |
| Files | |
|---|---|
| Instructions | German/English |
| MSDS | sdsRAE7071.zip (All languages) |