Pathogenic bacteria
Detect pathogens that can cause food poisoning
- Home
- /
- Analytes
- /
- Microbiology
- /
- Pathogenic bacteria
Pathogenic bacteria in food production: Risks, detection, and prevention
Understanding pathogenic bacteria in food safety
Pathogenic bacteria are harmful microorganisms that can contaminate food and cause serious illnesses in humans. These bacteria, including Salmonella, Listeria monocytogenes, Escherichia coli (E. coli), and Campylobacter, thrive in various food products and environments. Ensuring that food is free from dangerous bacteria is crucial for public health, as foodborne illnesses can lead to severe complications, hospitalizations, and even fatalities.
Sources and risks of pathogenic bacteria contamination
Food contamination can occur at multiple stages of the production chain, from raw materials to finished products. Some of the most common sources of pathogenic bacteria include:
- Raw meat and poultry – Can harbor Salmonella, E. coli, and Campylobacter if not handled or cooked properly.
- Dairy products – Unpasteurized milk and soft cheeses can contain Listeria and E. coli.
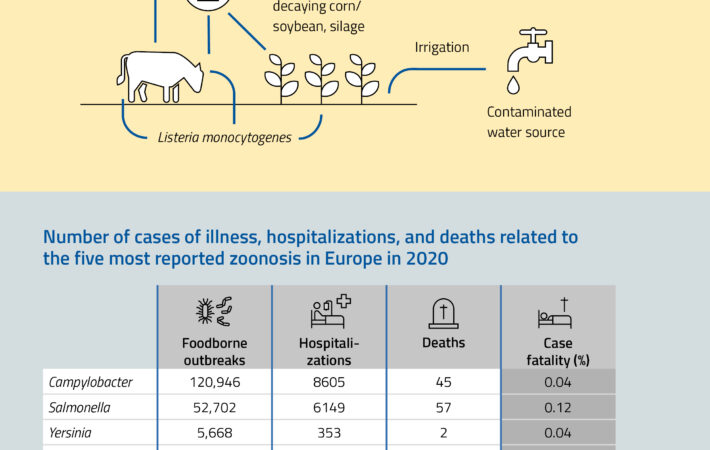
- Fresh produce – Fruits and vegetables can become contaminated through irrigation water or improper handling.
- Seafood – Shellfish and fish can carry Vibrio bacteria if sourced from contaminated waters.
- Processed and ready-to-eat foods – Cross-contamination during manufacturing or storage can introduce bacteria.
Given the widespread risks, stringent food safety measures are necessary to prevent bacterial growth and contamination.
Together with Salmonella, Campylobacteriosis is one of the most significant diarrheal diseases worldwide. The increase in Campylobacter enteritis is driven by the widespread presence of the bacterium in various wild and farm animals, as well as pets. As a commensal in the gut of poultry, Campylobacter enters the human food chain via these animals. Additionally, milk, ground meat, and drinking water can act as transmission pathways. Campylobacter is released in high quantities by numerous hosts, contaminating food and reaching humans. The infective dose for Campylobacter enteritis is low, requiring fewer than 500 bacteria to cause infection. Of the approximately 15 known Campylobacter species, C. jejuni and C. coli are the primary causes of human gastroenteritis.
Clostridium species are gram-positive, spore-forming bacteria present almost everywhere in the environment. Important pathogenic species include Clostridium botulinum, Clostridium perfringens, and Clostridium estertheticum. If food is not adequately cooled, it can become contaminated, leading to serious illnesses. These bacteria are heat-resistant and can survive in thoroughly heated food products.
Legionella are gram-negative, rod-shaped bacteria that live in water and are considered potential human pathogens. Legionella pneumophila is the species most responsible for human illnesses, causing Legionnaire’s disease (legionellosis). The disease is typically transmitted through contaminated drinking water.
Listeria bacteria are ubiquitous in the environment. Quantitative detection of Listeria can assess contamination risk and ensure preventive measures are taken if necessary. Listeria monocytogenes is a major cause of food poisoning and can lead to sepsis, meningitis, and encephalitis.
Salmonella is ranked among the most important initiators of cases of food poisoning. The WHO estimates more than 16 million global infections per year, and more than half a million cases are fatal. Salmonella are found in raw food such as eggs, meat and milk. The hazard potential is high, particularly in food that should only be slightly heated or not at all heated (e.g. raw milk cheese, raw sausage, chocolate, ice cream). In today’s mass animal farming industry, contaminations with Salmonella can hardly be avoided. Therefore, effective quality control with reliable test methods is important. For this purpose, we offer a series of different test systems, for the hygienic control of surfaces as well as for the analysis of food.
Staphylococcus aureus is a ball-shaped bacterium which can be observed as a grape-like clustered organism under the microscope. Cells are sized between 0.8 and 1.2 µm, Gram-positive and non-motile. S. aureus is a natural commensal of humans which lives on skin and upper airways and doesn’t cause diseases in general. In case of infections with pathogenic strains or in immunodeficient persons, S. aureus is able to cause skin infections and abscesses but also life-threatening diseases as pneumonia, meningitis, endocarditis, or toxic shock syndrome (TSS).
One reason for the facultative pathogenicity of S. aureus is its ability to form enterotoxins (SET), which can accumulate in contaminated food and cannot be inactivated totally by heat treatment. Contamination of foodstuffs with S. aureus is almost always caused by humans (hands, sneezing, coughing). S. aureus is therefore considered as indicator for poor personal hygiene.
Beside its relevance for food hygiene, S. aureus also plays a significant role in hospital hygiene. Especially strains of S. aureus which have acquired resistances against common antibiotics the so-called „methicillin resistant Staphylococcus aureus” (MRSA) might pose a non-assessable danger as they are difficult-to-treat.
The bacteria Escherichia coli (abbreviated E. coli) is a natural component of the intestinal flora in humans and animals and it is usually harmless. However, there are pathogenic strains that can cause serious infections. One of these pathogenic strains is known as enterohemorrhagic Escherichia E. coli (“EHEC”). These bacteria build the toxic substances verotoxin or shiga toxin and are also known as verotoxin-producing E. coli (“VTEC”) or shiga toxin-producing E. coli (“STEC”). Infections with STEC/VTEC are ranked as one of the most common food-related bacterial diseases that can even result in death. Sources of contamination mainly include raw meat and unpasteurized dairy products.
The group of Vibrio spp. includes pathogenic microorganisms which can cause serious infections after consumption of contaminated food. In diseases correlated to seafood, Vibrio spp. is one of the major causes of gastroenteritis, wound infection and sepsis. Additionally, in serious infections with the potential for epidemics such as Cholera, Vibrio spp. is causative.
The bacterium Vibrio parahaemolyticus naturally inhabits coastal waters and is present in higher concentrations during summer. The organism is halophilic or salt-requiring. Therefore, Vibrio parahaemolyticus is found in marine environments, seafood and the feces of patients with acute enteritis. Vibrio parahaemolyticus-associated gastroenteritis is the infection caused by this organism.
Importance of detecting and controlling pathogenic bacteria
To protect consumers and meet regulatory requirements, food manufacturers and quality assurance teams must proactively detect and control pathogenic bacteria in their production processes. Implementing advanced bacterial detection methods ensures food safety and helps prevent outbreaks. Contamination in food products can lead to costly recalls, legal issues, and reputational damage for brands.
Advanced testing solutions for pathogenic bacteria detection
R-Biopharm offers highly sensitive and specific test systems designed for the rapid and reliable detection of pathogenic bacteria in food production environments. These testing solutions provide critical advantages for food manufacturers and regulatory bodies:
- Rapid and reliable results – Fast detection of Listeria, Salmonella, E. coli, and other pathogens to prevent foodborne illnesses.
- Comprehensive monitoring – Tests for raw materials, finished products, food contact surfaces, and production environments.
- Easy integration into HACCP systems – Ensures food safety compliance with minimal workflow disruption.
- High sensitivity and specificity – Detects low bacterial levels to prevent contamination before products reach consumers.
- Regulatory compliance – Supports adherence to international food safety standards, including HACCP, ISO 17025, and FDA/EFSA guidelines.
Best practices for preventing pathogenic bacteria contamination
In addition to using advanced detection systems, food producers must adopt best practices to minimize contamination risks:
- Strict hygiene protocols – Regular handwashing, sanitization of equipment, and proper food handling procedures.
- Temperature control – Proper refrigeration and cooking temperatures to inhibit bacterial growth.
- Cross-contamination prevention – Separation of raw and cooked foods, proper storage, and dedicated food preparation areas.
- Water and environmental monitoring – Regular testing of processing water and facility surfaces for bacterial presence.
- Employee training and compliance – Continuous education on food safety protocols and hazard prevention.
Ensuring food Safety with R-Biopharm’s test systems
Foodborne pathogens pose significant health risks, making reliable detection and prevention strategies essential for the food industry. R-Biopharm’s test systems offer a robust solution to identify, quantify, and control pathogenic bacteria at every stage of food production. By integrating these tests into a comprehensive HACCP plan, food manufacturers can enhance consumer safety, maintain regulatory compliance, and protect their brand reputation.
For more information on our advanced bacterial detection solutions, contact R-Biopharm today and safeguard your food production against pathogenic bacteria.
Product portfolio
| Product | Description | No. of tests/amount | Art. No. |
|---|---|---|---|
| RIDASCREEN® SET Total (96 tests) |
RIDASCREEN® SET Total is a sandwich enzyme immunoassay for the combined detection of Staphylococcus aureus enterotoxins A, B, C, D and E in fluid and solid foods as well as in bacterial cultures. Read more |
Microtiter plate with 96 wells (12 strips with 8 breakable wells each), 96 determinations. |
R4105 |
| RIDASCREEN® SET A,B,C,D,E |
RIDASCREEN® SET A,B,C,D,E is a sandwich enzyme immunoassay for the identification of Staphylococcus enterotoxins A, B, C, D and E in fluid and solid foods as well as in bacterial cultures. Based on its sensitivity the RIDASCREEN® SET A,B,C,D,E test … Read more |
Microtiter plate with 96 wells (12 strips with 8 wells each), one strip is necessary for analysis of one sample, 12 tests in total. |
R4101 |
| Product | Description | No. of tests/amount | Art. No. |
|---|---|---|---|
| Compact Dry LM |
Usage of Compact Dry LM is a simple and safe test procedure for detection of Listeria monocytogenes in pre-enriched samples of foods. The ready-to-use plates consist of a special 50 mm diameter petri dish containing a detection specific nutrient pad. … Read more |
100 nutrient plates | HS9901 |
| Compact Dry PA |
Compact Dry PA is a simple and safe test procedure for the determination and quantification of Pseudomonas aeruginosa counts in foods, cosmetics, water samples or pharmaceutical materials. The ready-to-use plates consist of a special 50 mm diameter … Read more |
100 nutrient plates | HS9491 |
| Compact Dry BC |
Usage of Compact Dry BC is a simple and safe test procedure for determination and quantification of Bacillus cereus in foods or raw materials. The ready-to-use plates consist of a special 50 mm diameter petri dish containing a detection specific … Read more |
100 nutrient plates | HS9721 |
| Compact Dry X-SA |
Usage of Compact Dry X-SA is a simple and safe test procedure for determination and quantification of Staphylococcus aureus in foods, cosmetics or raw materials – as well as pharmaceutical raw materials. The ready-to-use plates consist of a special … Read more |
100 nutrient plates | HS9621 |
| Compact Dry VP |
Usage of Compact Dry VP is a simple and safe test procedure for detection and quantification of Vibrio parahaemolyticus as well as Vibrio spp. in foods or raw materials. The ready-to-use plates consist of a special 50 mm diameter petri dish … Read more |
100 nutrient plates | HS8821 |
| Compact Dry SL |
Usage of Compact Dry SL is a simple and safe test procedure for detection of Salmonella in pre-enriched samples of foods or raw materials. The ready-to-use plates consist of a special 50 mm diameter petri dish containing a detection specific nutrient … Read more |
100 nutrient plates | HS9401 |
| Compact Dry LS |
Usage of Compact Dry LS is a simple and safe test procedure for quantification of Listeria contaminations in foods or raw materials – as well as pharmaceutical raw materials. The ready-to-use plates consist of a special 50 mm diameter petri dish … Read more |
100 nutrient plates | HS8811 |
| Compact Dry EC |
Usage of Compact Dry EC is a simple and safe test procedure for determination and quantification of coliform bacteria and E. coli in foods, cosmetics, water or raw materials – as well as pharmaceutical raw materials. The ready-to-use plates consist … Read more |
100 nutrient plates | HS8781 |
| Compact Dry CF |
Usage of Compact Dry CF is a simple and safe test procedure for determination and quantification of coliform bacteria in foods, cosmetics or raw materials – as well as pharmaceutical raw materials. The ready-to-use plates consist of a special 50 mm … Read more |
100 nutrient plates | HS8791 |
| Product | Description | No. of tests/amount | Art. No. |
|---|---|---|---|
| SureFast® Enterobacteriaceae 4plex |
The SureFast® Enterobacteriaceae 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of specific DNA sequences of Enterobacteriaceae, Cronobacter spp. and Salmonella spp.. Read more |
100 reactions | F5180 |
| SureFast® Fecal Screen 4plex |
The SureFast® Fecal Screen 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of specific DNA sequences of Enterobacterales, Enterococci, Escherichia coli and Shigella spp. in water. Read more |
100 reactions | F5504 |
| SureFast® Listeria 3plex ONE |
The SureFast® Listeria 3plex ONE is a multiplex real-time PCR test kit for the fast and simple detection of Listeria spp. and Listeria monocytogenes in different types of food and environmental samples. Benefits: MicroVal – 2023LR114 AOAC OMA … Read more |
100 reactions | F5217 |
| SureFast® Campylobacter 4plex |
The SureFast® Campylobacter 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of specific DNA sequences of Campylobacter jejuni, Campylobacter lari and Campylobacter coli. Read more |
100 reactions | F5170 |
| SureFast® STEC 4plex ONE |
The SureFast® STEC 4plex ONE can be applied for the fast and simple isolation, detection and differentiation of specific DNA sequences of the Escherichia coli virulence factors stx1 (subtype a-d), stx2 (subtype a-g), eae and the Escherichia coli … Read more |
100 reactions | F5265 |
| SureFast® MRSA 4plex |
The SureFast® MRSA 4plex is a real-time PCR for the direct, qualitative detection and differentiation of methicillin-resistant Staphylococcus aureus (MRSA). The test detects the SCCmec/orfX junction, the mecA/mecC-gene and a specific DNA sequence of … Read more |
100 reactions | F7117 |
| SureFast® Yersinia 3plex |
The SureFast® Yersinia 3plex is a real-time PCR for the direct, qualitative detection and differentiation of specific ail gene DNA sequences of Yersinia pseudotuberculosis and Yersinia enterocolitica. Read more |
100 reactions | F5132 |
| SureFast® Escherichia coli Serotype II 4plex |
The SureFast® Escherichia coli Serotype II 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of specific wzx gene DNA sequences of Escherichia coli O45, wbdl gene DNA sequences of Escherichia coli O111 and … Read more |
100 reactions | F5168 |
| SureFast® Escherichia coli Serotype I 4plex |
The SureFast® Escherichia coli Serotype I 4plex is a multiplex real-time PCR kit for the direct, qualitative detection and differentiation of specfifc wzx gene DNA sequences of Escherichia coli O121, O26 and O103. Read more |
100 reactions | F5167 |
| SureFast® Foodborne Pathogens 4plex |
The SureFast® Foodborne Pathogens 4plex is a multiplex real-time PCR for for the direct, qualitative detection and differentiation of Escherichia coli virulence factors (stx1 [subtype a-d], stx2 [subtype a-g]), Listeria monocytogenes and Salmonella … Read more |
100 reactions | F5175 |
| SureFast® Salmonella Species/Enteritidis/Typhimurium 4plex |
The SureFast® Salmonella Species/Enteritidis/Typhimurium 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium. Each reaction contains an … Read more |
100 reactions | F5166 |
| SureFast®STEC Screening PLUS |
The SureFast® STEC Screening PLUS is a real-time PCR for the direct, qualitative detection of DNA sequences of the virulence factors stx1 (subtype a-d) and stx2 (subtype a-g) of Escherichia coli (STEC). Each reaction contains an internal … Read more |
100 reactions | F5105 |
| SureFast® Parasitic Water Panel 4plex |
The SureFast® Parasitic Water Panel 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of Giardia intestinalis, Entamoeba histolytica and Cryptosporidium spp.. Each reaction contains an internal … Read more |
100 reactions | F5506 |
| SureFast® Legionella 3plex |
The SureFast® Legionella 3plex is a real-time PCR for the direct, qualitative detection of Legionella spp. and Legionella pneumophila. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5505 |
| SureFast® Vibrio 4plex |
The SureFast® Vibrio 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5161 |
| SureFast® Pseudomonas aeruginosa PLUS |
The SureFast® Pseudomonas aeruginosa PLUS is a real-time PCR for the direct, qualitative detection of a specific DNA sequence of Pseudomonas aeruginosa. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5503 |
| SureFast® Escherichia coli PLUS |
The test detects Escherichia coli DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5157 |
| SureFast® Cronobacter sakazakii PLUS |
SureFast® Cronobacter sakazakii PLUS is a real-time PCR for the direct, qualitative detection of Cronobacter sakazakii DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5115 |
| SureFast® Emetic Bacillus cereus PLUS |
The SureFast® Emetic Bacillus cereus PLUS is a real-time PCR for the direct, qualitative detection of a specific cereulide synthetase DNA sequence of the emetic Bacillus cereus. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5127 |
| SureFast® Clostridium perfringens PLUS |
The SureFast® Clostridium perfringens PLUS is a real-time PCR for the direct, qualitative detection of a specific alpha-toxin DNA sequence of Clostridium perfringens. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5123 |
| SureFast® Clostridium botulinum Screening PLUS |
The SureFast® Clostridium botulinum Screening PLUS is a real-time PCR for the direct, qualitative detection of specific Botulinum neurotoxins (BoNT) A, B, E and F DNA sequences of Clostridium botulinum, Clostridium baratii and Clostridium butyricum. … Read more |
100 reactions | F5110 |
| SureFast® Clostridium estertheticum PLUS |
The test detects Clostridium estertheticum DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5160 |
| SureFast® Salmonella ONE |
Salmonella spp. detection with real-time PCR Two in one – Salmonella test kit with included DNA preparation The kit is intended to be used for the fast and simple isolation and detection of Salmonella DNA from enrichment cultures. Each reaction … Read more |
100 reactions | F5211 |
| SureFast® Salmonella PLUS |
The SureFast® Salmonella PLUS is a real-time PCR for the direct, qualitative detection of a specific DNA sequence of Salmonella spp.. The SureFast® Salmonella PLUS real-time PCR kit has been validated and certified in combination with the … Read more |
100 reactions | F5111 |
| SureFast® EHEC/EPEC 4plex |
SureFast® EHEC/EPEC 4plex is a multiplex real-time PCR for the direct, qualitative detection and differentiation of DNA sequences of the virulence factors stx1 (subtype a-d), stx2 (subtype a-g), eae and the E. coli and Shigella spp. virulence factor … Read more |
100 reactions | F5128 |
| SureFast® Legionella Screen PLUS |
The test detects Legionella spp. DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5502 |
| SureFast® Bacillus cereus group PLUS |
The SureFast® Bacillus cereus group PLUS is a real-time PCR for the direct, qualitative detection of specific DNA sequences of Bacillus cereus group (Bacillus anthracis, Bacillus cereus, Bacillus cytotoxis, Bacillus mycoides, Bacillus … Read more |
100 reactions | F5126 |
| SureFast® Staphylococcus aureus PLUS |
The SureFast® Staphylococcus aureus PLUS is a real-time PCR for the direct, qualitative detection of a specific Staphylococcus aureus DNA sequence. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5116 |
| SureFast® Listeria monocytogenes PLUS |
SureFast® Listeria monocytogenes PLUS is a real-time PCR for the direct, qualitative detection of DNA sequences of the Listeria monocytogenes virulence-associated gene prfA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5113 |
| SureFast® Listeria Screening PLUS |
The SureFast® Listeria Screening PLUS is a real-time PCR for the direct, qualitative detection of a specific DNA sequence of Listeria spp.. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5117 |
| SureFast® Legionella pneumophila PLUS |
The test detects Legionella pneumophila DNA. Each reaction contains an internal amplification control (IAC). Read more |
100 reactions | F5501 |